Chemical Properties of Phenols
Chemical Properties of Phenols: Overview
This Topic covers sub-topics such as Nitration of Phenols, Chemical Properties of Phenols, Reaction of Phenol with NaOH, Kolbe's Reaction of Phenol, Reaction of Phenol with Zinc Dust, Acidic Character of Phenols and, Halogenation of Phenols
Important Questions on Chemical Properties of Phenols
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Phenols give and nitrophenol on nitration with conc. and mixture.
Reason : group in phenol is directing.
Assertion : Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason : In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
Electrophilic substitution involved in _____ of phenol.
At high temperature, phenol produces para product on sulphonation.
What is sulphonation reaction of phenol?
Write the reaction of phenol with sulphuric acid.
The reaction sequence,
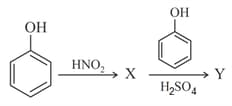
the major products and , respectively, are
Reaction of phenol with followed by heating with under high pressure, and subsequent acidification gives compound as the major product, which can be purified by steam distillation. When reacted with acetic anhydride in the presence of a trace amount of conc. compound produces as the major product. Compound is
Phenol on sulphonation at gives
Give structures and names of the principal organic products expected from the monosulfonation of:
salicylaldehyde
Phenol can be distinguished from ethanol by the reactions with _____.
What is the action of following reagent on phenol?
Concentrated sulphuric acid at room temperature.
What is the action of following reagent on phenol?
Dilute nitric acid at room temperature.
Name the reagent used to convert phenol into o-Phenolsulphonic acid.
Name the reagent used to convert Phenol into -Tribromophenol.
Explain the following reaction of phenol.
Kolbe's reaction.
Explain the following reaction of phenol.
Reimer-Tiemann reaction.
Explain the following:
o-Nitrophenol is more acidic than o-Cresol.
Explain the following:
Phenol is more acidic than ethanol.
Which is stronger acid in the following pair ?
-Nitrophenol and -Trinitrophenol
